Although some may disagree, most believe that the term “pH” refers to “percentage of hydrogen”. As we all know, water is H20, which is two parts hydrogen and one part oxygen. Only products that are water soluble have a pH rating. We get confused some times when water is referred to as the “universal solvent” and then refer to other solvents that are petroleum based.
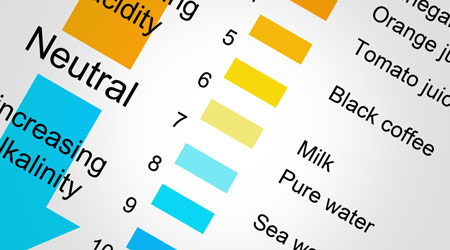
pH plays a significant role in cleaning. The pH scale goes from 0 (very strong acid) to 14 (very strong alkaline) with 7 being neutral. The scale is logarithmic, meaning that a chemical with a pH of 8 is 10 times the alkalinity of one of 7. A pH of 9 would indicate an alkalinity that is 100 times more alkaline than a pH of 7, and so on. 0 pH and 14 pH are 10,000,000 times stronger than 7 which means such products are very corrosive and caustic. It is important to use extreme care in mixing such products, since it can result in a catastrophic reaction resulting in injury and damage to any surfaces impacted.
Generally speaking, the further away from pH of 7, the more powerful the cleaner, which means that both highly alkaline and highly acidic products can be dangerous and potentially explosive if used incorrectly. The limiting factor is the type of soil and the surface being cleaned. Most organic soils are slightly acidic (6.9 – 5.0), so we use cleaners that are slightly alkaline (7.1-8.5). The rule of thumb is to always use the mildest first (water) then progress with a stronger product as necessary based on the type soil involved. Learn to use a pH strip to determine what you are dealing with. Remember that only “aqueous” (water) based products have a pH reading and that oil based solvents do not have a pH since they do not mix with water.
Your comments and questions are important. I hope to hear from you soon. Until then, keep it clean...
Mickey Crowe has been involved in the industry for over 35 years. He is a trainer, speaker and consultant. You can reach Mickey at 678-314-2171 or CTCG50@comcast.net
posted on 9/2/2016

 The Down and Dirty on Cleaning in Virus Season
The Down and Dirty on Cleaning in Virus Season How Surfactant Use is Expanding in Commercial Cleaning
How Surfactant Use is Expanding in Commercial Cleaning Maximize Your Margins: Learn How to Automate Pricing and Track Rebates
Maximize Your Margins: Learn How to Automate Pricing and Track Rebates