Cambridge University Press released a study that evaluated the efficacy of automated disinfected dispenser systems in hospitals. The results of the study — which oversaw 10 unique hospitals and four additional healthcare systems across five states — highlighted the importance of improved dispenser monitoring to ensure the correct concentrations of disinfectants are dispensed. Here is the full report:
Abstract
Automated dispensers that dilute concentrated disinfectants with water are commonly used in healthcare facilities. In a point-prevalence product evaluation, nine of 10 (90 percent) hospitals using dilutable disinfectants had one or more malfunctioning dispensers. Twenty-nine of 107 (27.1 percent) systems dispensed product with lower-than-expected concentrations, including 15 (14 percent) with no detectable disinfectant.
Introduction
Environmental services (EVS) and infection prevention personnel consider factors such as efficacy, wet-contact and kill times, safety, ease of use, and cost when selecting cleaning and disinfection products. Many products are distributed as a concentrate that is diluted to in-use concentrations. The use of dilutable products may reduce costs and can be beneficial for disinfectants that are more stable as a concentrate than at in-use concentrations.
Dilutable disinfectants are often dispensed from automated wall-mounted systems that mix concentrated disinfectant and water. However, monitoring is required to ensure that the dispensers are working correctly. Boyce et al. found variations in the concentration of a dilutable quaternary ammonium disinfectant delivered by an automated dispenser; the issues were resolved after installation of water-pressure regulators and modifications of the flow-control devices in concentrate containers. It was recommended that hospitals utilizing dispensing stations conduct periodic testing to verify that appropriate concentrations are being dispensed. Others have demonstrated that automated systems sometimes deliver lower-than-predicted disinfectant concentrations. In a culture survey, we found high rates of surface contamination after completion of post-discharge cleaning and disinfection in some hospitals using automated dispensers.
Therefore, we conducted an evaluation of automated dispensing systems in several hospitals.
Methods
The study protocol was approved by the Cleveland VA Medical Center’s Research and Development Committee. We conducted a point-prevalence evaluation of automated disinfectant dispensing systems in a convenience sample of 10 hospitals from four healthcare systems in five states. For a minimum of five medical-surgical wards and/or intensive care units, we collected 10mL disinfectant samples from dispensers and buckets of in-use disinfectant. The disinfectants included quaternary ammonium disinfectant cleaners, and a peracetic acid/hydrogen peroxide product.
For the peracetic acid product, pH was measured with Micro Essential Lab Single-Roll Hydrion pH Test Paper. Peracetic acid concentrations were also measured using a dropper-bottle method with a lower limit-of-detection of 300 ppm. The expected in-use concentration of peracetic acid is roughly 1300 ppm (0.13 percent) with pH roughly 3; 8 samples with more than1,800 ppm were considered to have higher-than-expected concentrations.
For the peracetic acid product, the manufacturer recommends quarterly calibration of the systems and provides posters with user instructions (Supplementary material). The instructions include guidance to check a Green/Red low product indicator for when the concentrate bottle should be changed (i.e., change when indicator window shows three-fourths red) and an optional intermittent pH check.
For the quaternary ammonium disinfectants, quaternary ammonium concentrations were measured using Micro Essential Lab Hydrion Quaternary Test Paper Kits; the expected in-use concentrations of the products are roughly 700–800 parts per million (ppm) with pH 8–9.
To assess the efficacy of samples with lower-than-expected disinfectant levels, the American Society for Testing and Materials (ASTM) standard quantitative carrier disk test method was used with five percent fetal calf serum as soil load. The test organisms included a clinical methicillin-resistant Staphylococcus aureus (MRSA) strain for the quaternary ammonium product and Clostridioides difficile American Type Culture Collection strain 43598 for the peracetic acid product. The exposure times were 5 and 10 minutes for the peracetic acid and quaternary ammonium disinfectants, respectively.
For one hospital using the quaternary ammonium product, additional point-prevalence evaluations were conducted after EVS implemented interventions that included increased monitoring of quaternary ammonium concentrations of dispensed product. The supplementary material provides details on the intervention.
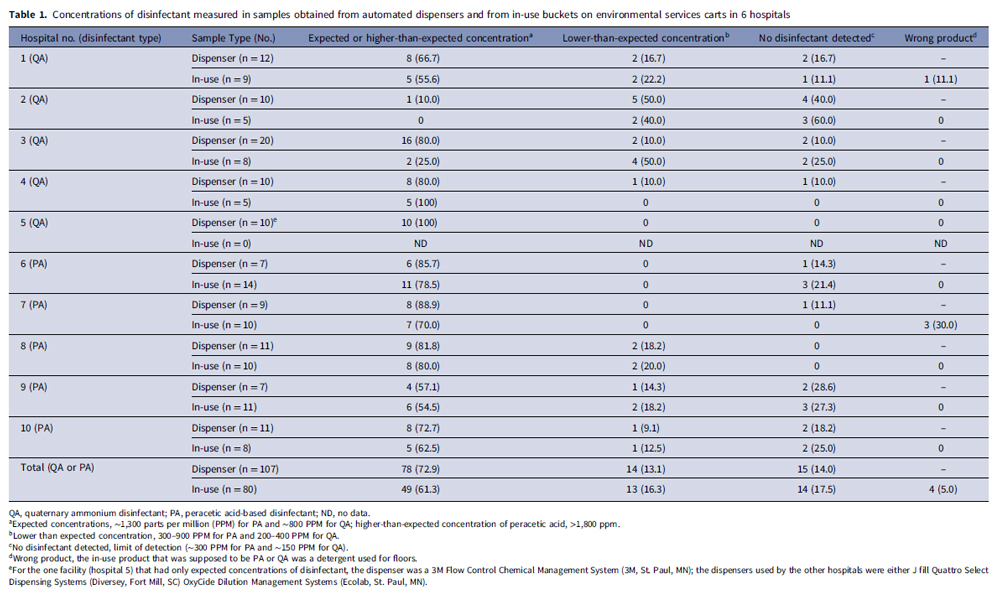
None of the hospitals reported conducting routine monitoring of disinfectant dispensers. Nine of 10 (90 percent) hospitals had one or more systems dispensing lower-than-expected disinfectant concentrations, and 8 (80 percent) had dispensers that delivered product with no detectable disinfectant (See Table 1). Overall, 29 of 107 (27.1 percent) systems dispensed product with lower-than-expected concentrations, including 15 (14 percent) with no detectable disinfectant.
Of 45 samples of peracetic acid product, 26 (57.8 percent) had higher-than-expected peracetic acid concentrations (≥1800 ppm; peak 2,100 ppm), 9 (20 percent) had expected concentrations (1,200–1,500 ppm), 4 (8.9 percent) had lower-than-expected concentrations (300–900 ppm), and 6 (13.3 percent) had undetectable concentrations. pH measurements distinguished dispensers with expected or higher-than-expected peracetic acid disinfectant concentrations (pH around 3) versus those with lower-than-expected concentrations (pH 3.5–5) versus no detectable disinfectant (pH around 6).
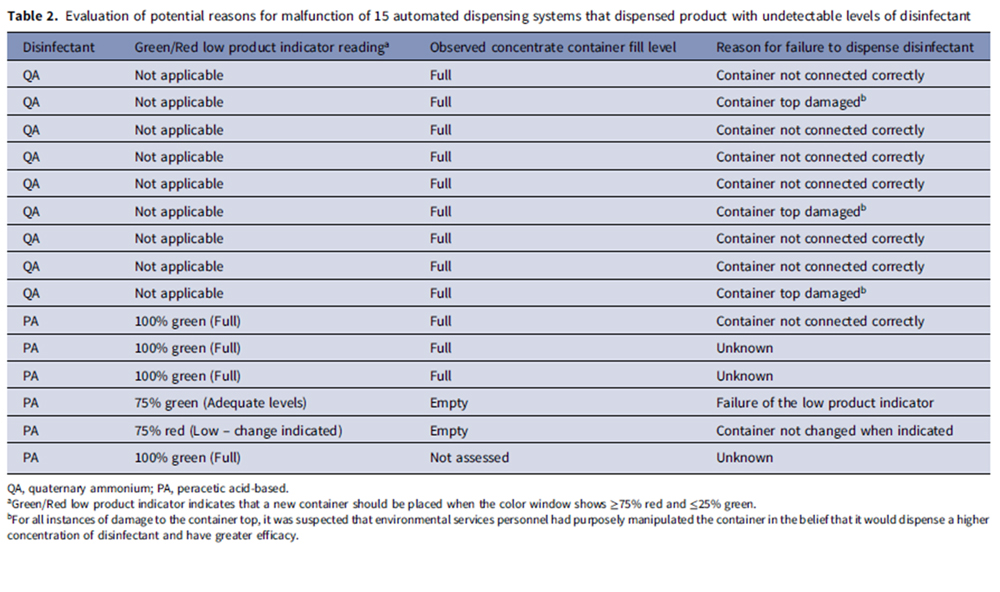
A second table (See Table 2) shows reasons for malfunction of the 15 systems that dispensed undetectable levels of disinfectant. The identified reasons included concentrate container not being connected correctly (n = 7), concentrate container top being damaged (n = 3), low product indicator not functioning correctly resulting in use of an empty concentrate container (n = 1), and personnel not changing the container when the low product indicator indicated that a change was due (n = 1). In three cases, the reason for the malfunction was not clear.
Of 80 in-use disinfectant samples obtained from EVS carts, 27 (33.8 percent) had lower-than-expected disinfectant concentrations, including 14 (17.5 percent) with no detectable disinfectant. One sample with no detectable disinfectant was from a functioning dispenser but the EVS worker acknowledged not obtaining fresh product for days.
Of nine employees using product with no detectable disinfectant who were interviewed, three had not noticed that the product was incorrect, whereas six noticed that the product did not smell or appear right but had not notified their supervisor. In four instances, in-use products that EVS personnel identified as disinfectants were dilutable detergents intended for floors.
ASTM testing demonstrated that samples with ≤900 ppm of peracetic acid and ≤400 ppm of quaternary ammonium disinfectant resulted in <3 log10 colony-forming unit reductions in C. difficile spores and MRSA, respectively.
For the hospital using the quaternary ammonium product that conducted an intervention, initial testing after the intervention demonstrated that 1 of 12 dispensers delivered no detectable disinfectant. However, in two subsequent assessments, product from all dispensers had the expected disinfectant concentrations.
Discussion
Regular monitoring is essential to ensure that automated disinfectant dispensing systems are functioning and being used correctly. In the current study, nine of 10 study hospitals had at least one dispenser delivering lower-than-expected disinfectant concentrations, and 14 percent of the dispensed product had no detectable disinfectant. Failure to dispense any disinfectant was usually attributable to human error (i.e., container not connected correctly or damaged, container not changed when indicated). However, in one instance the low product indicator did not function correctly resulting in dispensing product with no disinfectant and in three instances the reason for failure was unclear.
Our findings suggest that there is an urgent need for improved monitoring of automated disinfectant dispensers. Regular monitoring of pH or disinfectant concentrations might be helpful to ensure that dispensers are functioning and being used correctly. At a minimum, we recommend testing when a new container of concentrate is placed in the dispenser and if the dispensed product does not smell or appear appropriate.
For the peracetic acid product, 58 percent of systems dispensed higher-than-expected peracetic acid concentrations (≥1800 ppm). Others have reported that automated systems sometimes dispense higher-than-expected peracetic acid concentrations. Additional evaluations are needed to assess whether the higher-than-expected concentrations pose a risk to EVS personnel.
Our study has some limitations. Only 10 hospitals were included and not all dispensers were tested. The effect of an intervention that included monitoring was only assessed in one facility. Additional studies are needed to identify effective approaches to ensure that dispensers function correctly and are monitored regularly.
Supplementary material. The supplementary material for this article can be found at https://doi.org/10.1017/ice.2024.148.
Acknowledgments. We thank the Environmental Management Services team at the Louis Stokes Cleveland VA Medical Center for helpful discussions and for assistance with the intervention.
Financial support. Supported by the Department of Veterans Affairs.
Competing interests. C.J.D has received research grants from Clorox and Pfizer. All other authors report no conflicts of interest relevant to this article.
References
1. Donskey CJ. Does improving surface cleaning and disinfection reduce health care-associated infections? Am J Infect Control 2013;41:S12–9.
2. Rutala WA, Weber DJ. Selection of the ideal disinfectant. Infect Control Hosp Epidemiol 2014;35:855–865.
3. Deshpande A, Mana TS, et al. Evaluation of a sporicidal peracetic acid/hydrogen peroxide-based daily disinfectant cleaner. Infect Control Hosp Epidemiol 2014;35:1414–6.
4. Cadnum JL, Jencson AL, O’Donnell MC, Flannery ER, Nerandzic MM, Donskey CJ. An increase in healthcare-associated Clostridium difficile infection associated with use of a defective peracetic acid-based surface disinfectant. Infect Control Hosp Epidemiol 2017;38:300–305.
5. Boyce JM. Modern technologies for improving cleaning and disinfection of environmental surfaces in hospitals. Antimicrob Resist Infect Control 2016;5:10.
6. Boyce JM, Sullivan L, Booker A, Baker J. Quaternary ammonium disinfectant issues encountered in an environmental services department. Infect Control Hosp Epidemiol 2016;37:340–2.
7. O’Neill C, Ramage L, Wyatt L, Ballantyne L. Quality control is indispensable for automated dilution systems with accelerated hydrogen peroxide. Can J Infect Control 2009;24:226–8.
8. United States Environmental Protection Agency. OxyCideTM Daily Disinfectant Cleaner. Available at: https://www3.epa.gov/pesticides/chem_search/ppls/001677-00237-20191003.pdf. Accessed March 15, 2024.
9. ASTM International, Designation E2197. Standard Quantitative Disk Carrier Test Method for Determining Bactericidal, Virucidal, Fungicidal, Mycobactericidal, and Sporicidal Activities of Chemicals. West Conshohocken, PA: ASTM International; 2011.
10. Hawley B, Casey M, Cummings KJ, Edwards N, Johnson A, Cox-Ganser J; National Institute of Occupational Safety and Health. Evaluation of exposure to a new cleaning and disinfection product and symptoms in hospital employees. NIOSH health hazard evaluation report, HHE 2015-0053-3269, 2017. Available at: https://stacks.cdc.gov/view/cdc/44556. Accessed March 15, 2024.
Authored by Jennifer L. Cadnum BS, Claire E. Kaple BS, Elizabeth C. Eckstein RN, Elie A. Saade MD, Amy J. Ray MD, Trina F. Zabarsky RN, Bernardino J. Guerrero BS, Mohamed H. Yassin MD, and Curtis J. Donskey MD, this original study titled “Dilution dysfunction: evaluation of automated disinfectant dispenser systems in 10 hospitals demonstrates a need for improved monitoring to ensure that correct disinfectant concentrations are delivered” was published in Infection Control & Hospital Epidemiology, volume 45, issue 11, pages 1362-1365, 2024 © Cambridge University Press, reproduced with permission.

 The Down and Dirty on Cleaning in Virus Season
The Down and Dirty on Cleaning in Virus Season How Surfactant Use is Expanding in Commercial Cleaning
How Surfactant Use is Expanding in Commercial Cleaning Maximize Your Margins: Learn How to Automate Pricing and Track Rebates
Maximize Your Margins: Learn How to Automate Pricing and Track Rebates